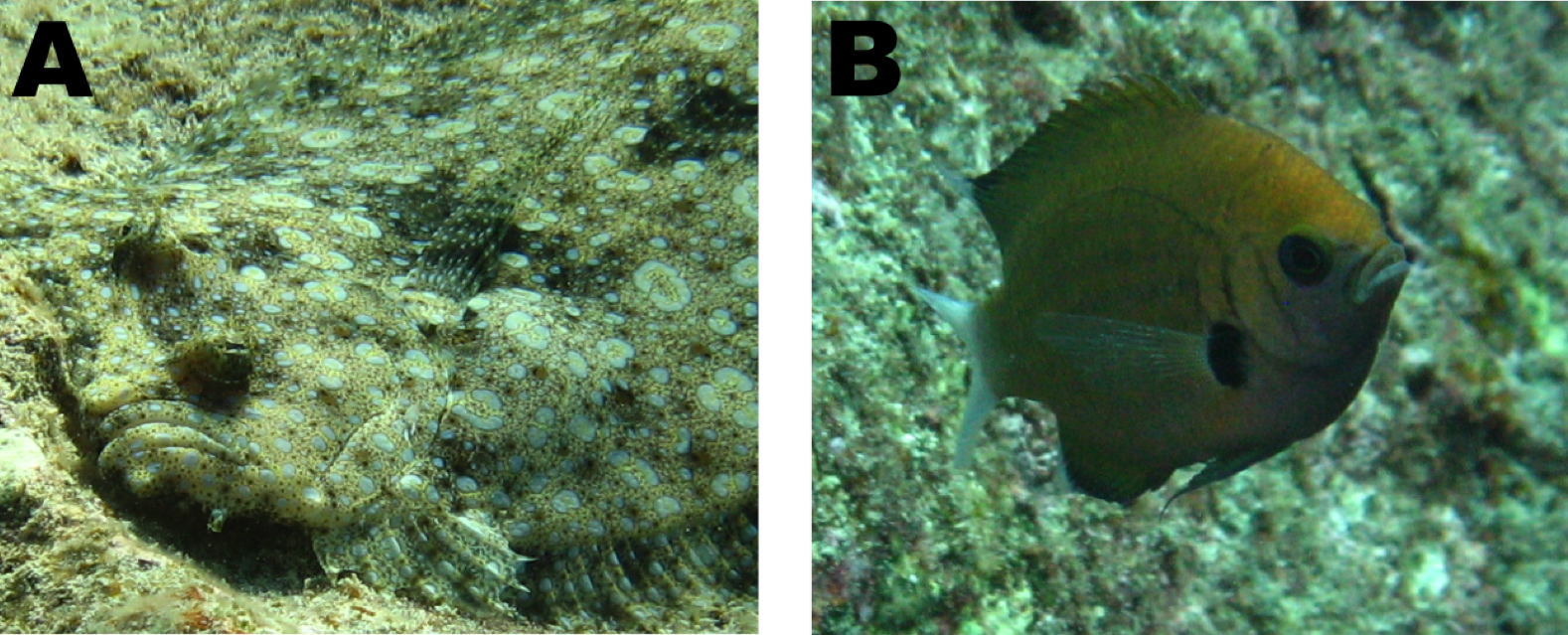
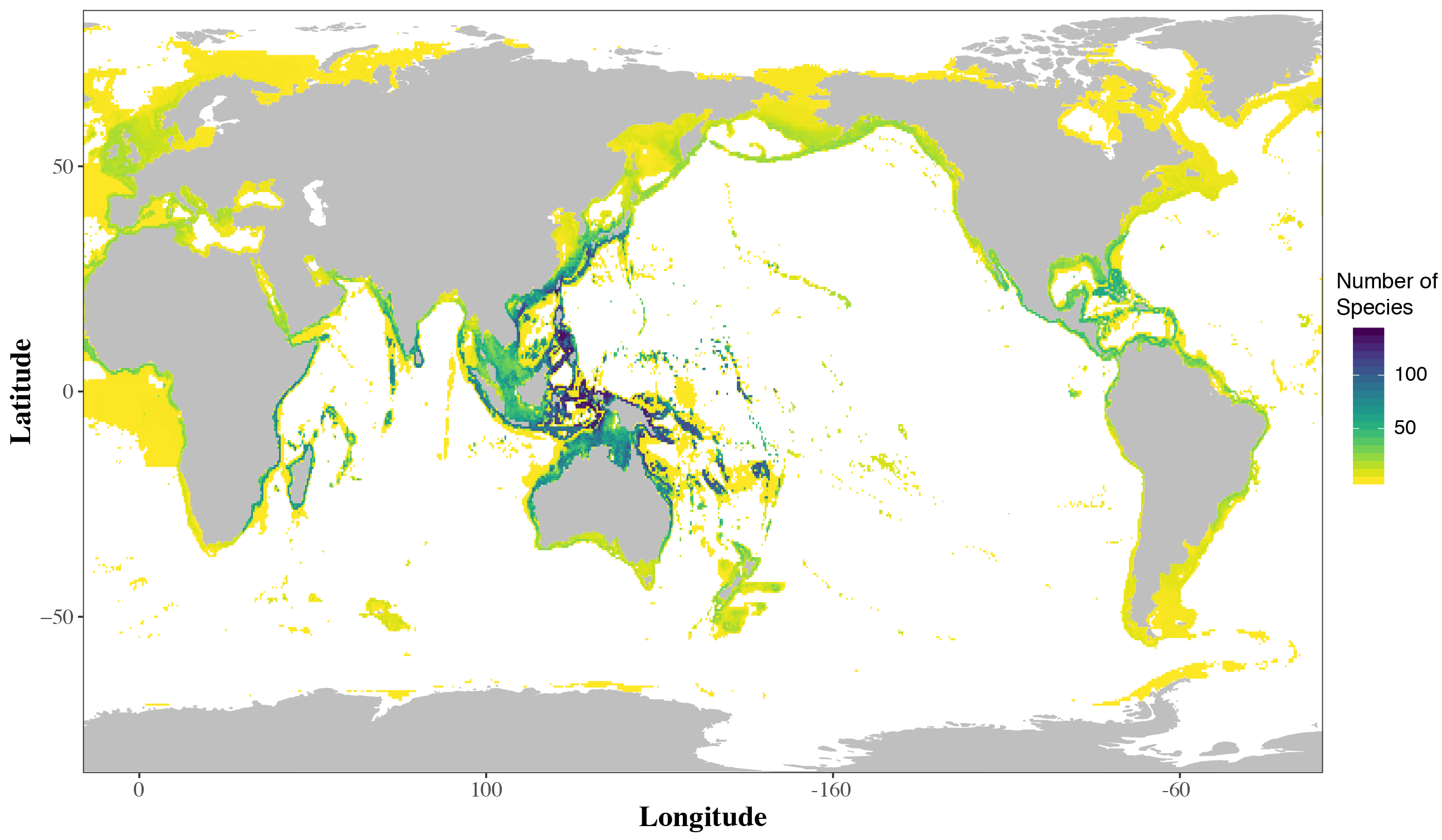
Flatfishes, the subject of my Fulbright fellowship, are a striking group of fishes with a distinct body plan. In the more than 850 species of flatfishes, both eyes are found on the same side of the head (Figure 1). These are iconic fishes of the Northern Hemisphere. Halibut and flounder are well known to the general public. Commercial and personal fishing efforts target these fishes in the cool waters of North America, Europe and Asia. However, most flatfishes are actually quite small and they may be found circumglobally in marine waters (Figure 2). Not restricted to near-shore marine habitats, flatfishes may be found from the deep sea to freshwaters from the tropics to the poles. Scientific study, though, has focused on large-bodied flatfishes of cool marine waters important in fisheries. Therefore, a significant research bias has affected flatfish research and many questions in flatfish biology have persisted (Gibson et al., 2015; Hensley, 1997). My Fulbright fellowship addressed key questions in flatfish biology by increasing the sampling of flatfish lineages from the tropics in both deep sea, coastal marine and freshwater habitats.
Taiwan is well situated near the center of flatfish diversity and my host, Professor Wei-Jen Chen of the Institute of Oceanography at National Taiwan University, has conducted several biodiversity expeditions in the tropics that have explored the broad diversity of flatfish lineages (Figure 3). With these samples, we constructed a DNA sequence data set to investigate a paradox in flatfish biology and to refine taxonomic classification of these fishes.
1. A Paradox in Flatfish Biology
While recent diversity (i.e. living species) of flatfishes is centered in the tropics, flatfish historic diversity (i.e. fossils) is centered around the Mediterranean Sea (Figure 4). During my fellowship I used quantitative methods to examine if the core diversity of flatfishes in the Central Indo-West Pacific is (1) a result of flatfish lineages accumulating in the Central Indo-West Pacific, or (2) evidence that the Central Indo-West Pacific is the area of origin for flatfishes.
Flatfish fossils suddenly appear in the fossil record as what are known as “crown” lineages. That is, ancient flatfish fossils in the 40 – 50 million years range exhibit anatomical characteristics like those of living flatfishes and may belong to the same taxonomic families. In order to resolve the paradox of flatfish diversity, the relationships between key flatfish fossils needed to be determined. How closely related are these fossils to extant flatfish lineages? In collaboration with Dr. Bruno Chanet (French Natural History Museum, Paris) an anatomically based data matrix was made that, when combined with molecular data, places flatfish fossils among living flatfish lineages, generating a time-calibrated hypothesis of flatfish diversification created (results not shown). The time-calibrated hypothesis of flatfish diversification allows reconstruction of ancestral distributions and temperature preferences, pointing to a tropical Indo-West Pacific origin for flatfishes approximately 55 million years ago.
The abundance of flatfish species in the Indo-West Pacific is most likely because this region is the area of origin of flatfishes.
2. Insights in Flatfish Taxonomy
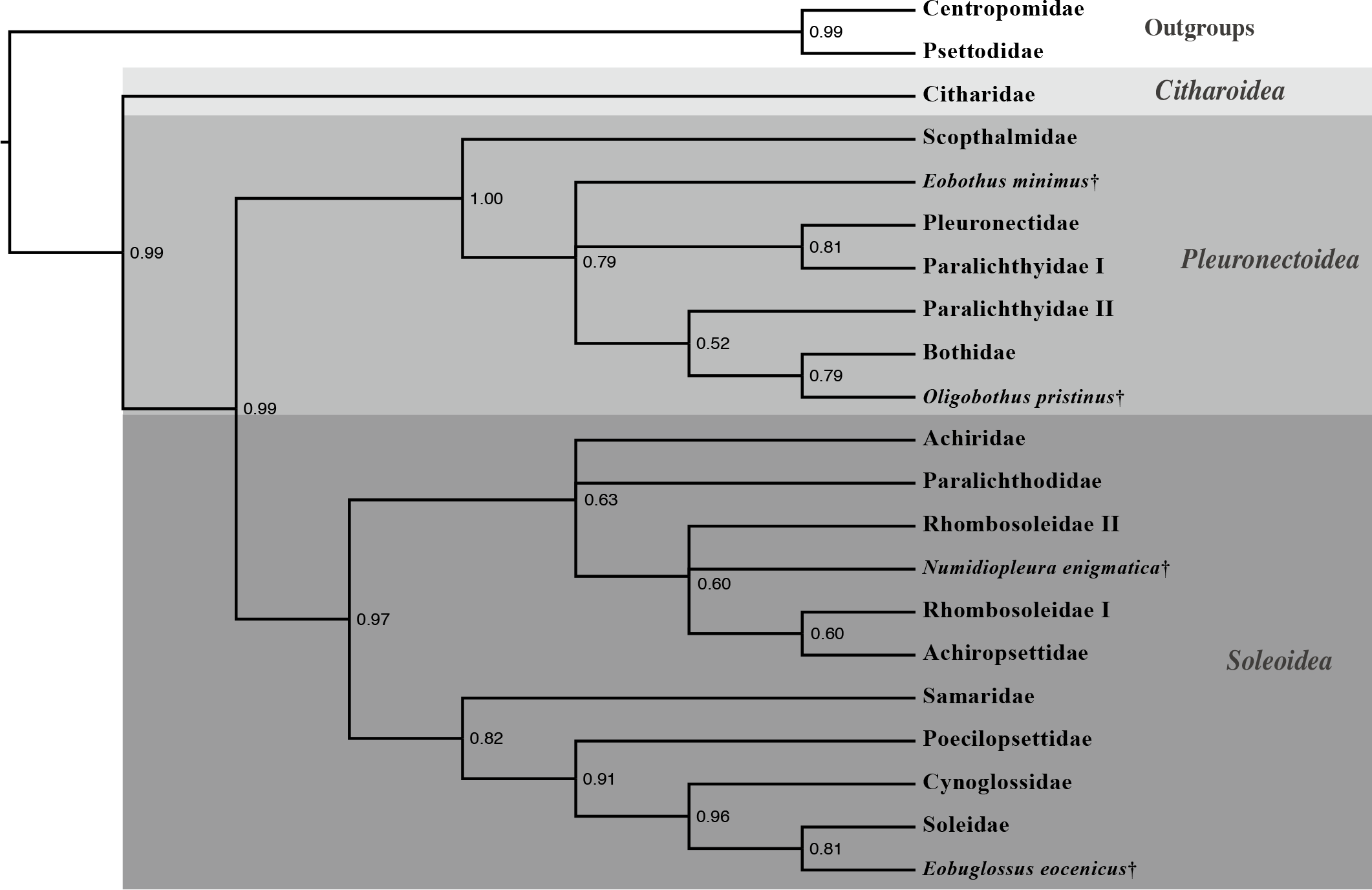
Flatfish alpha and beta taxonomy are active areas of research. Alpha taxonomy refers to species taxonomy and beta refers to a higher level (e.g. families). This project did not examine species diversity, but instead looked at taxonomic families of flatfishes and whether or not hypothesized superfamilies are realistic (Nelson, 2006). The results of this Fulbright fellowship support three superfamilies of flatfishes as described by Nelson (2006) and the creation of two new flatfish families. We evaluated the relationships of several fossils and found that two fossil flatfishes examined are representatives of extinct family-level diversity that have no known living examples. Thus, extinctions of high-level diversity of flatfishes have occurred in the past.
2.1 Flatfish family “Cyclopsettidae”
Molecular study previously had hinted that the family Paralichthyidae may contain two independent lineages (e.g. Pardo et al. 2005) and anatomical evidence had previously also indicated two very distinct groupings within Paralichthyidae (Hensley and Ahlstrom, 1984). My Fulbright research helps demonstrate that Paralichthyidae contains two distantly related lineages, and support the creation of a new family –the Spotfin Flounders “Cyclopsettidae” –composed of three genera (Cyclopsetta, Etropus and Citharichthys).
2.2 Flatfish family “Oncopteridae” and the Arctic flounders
Our analyses indicate that there is a lineage of Southern Hemisphere flatfishes composed of three families. The remo flounder (Oncopterus darwini) has long been considered a member of the family Rhomobosoleidae (‘turned soles’). Other rhombosoleids, however, are more closely related to the enigmatic Arctic flounders (Achiropsettidae). The remo flounder is distinct from other rhombosoleids as it is found in the southwest Atlantic Ocean and the other rombosoleids are focused around Australia and New Zealand (Nelson, 2006). Furthermore, this lineage is estimated to have been independent for 36.51 million years (Campbell et al., Submitted). We therefore have concluded that the remo flounder belongs in its own taxonomic family “Oncopteridae” (‘nail-fin’ flounders) and is closely related to Rhombosoleidae and Achiropsettidae.
2.3 Total-evidence flatfish classification
A total-evidence hypothesis of flatfish relationships is presented in Figure 5 showing the placement of key fossil flatfishes among extant lineages.
3. Concluding Thoughts
While Taiwan has been an ideal place to accumulate distinctive samples, the key lineage of the Chinese brill (Tephrinectes sinensis) was not represented despite its distribution in Taiwanese coastal waters and our efforts to collect it. It is very likely that this fish belongs to a new family composed of a single species (like Paralichthodidae and “Oncopteridae”) (Hoshino, 2001). Unfortunately, no molecular sequence data is available for this species and no tissue samples were available to us, and we could not include it in our study. Taxonomic research like that in this project has various applications and broad utility from combating invasive species to bioprospecting for pharmaceuticals. Flatfishes are major species in fisheries and aquaculture with taxonomic research leading to more effective management of fisheries and which may be applied to aquaculture. As demands on wild fisheries are ever increasing and with an expansion of aquaculture, basic taxonomy of fishes is as relevant as ever. New flatfishes continue to be described – 27 in the last 10 years – and the examination of new species and previously described species with molecular techniques are sure to reveal not only new species but also lead to changes in the higher-level classification of flatfishes.
Figure 1 – (A) A view of a flatfish showing the cranial asymmetry of the group, where both eyes sit on one side of the head. (B) A more typical cranial morphology of fishes, a damselfish


Bibliography
Agassiz, L., 1839. Recherches sur les Poissons fossiles. Imprimerie de Petitpierre, Neuchatel.
Baciu, D.S., Chanet, B., 2002. Les Poissons plats fossiles (teleostei: Pleuronectiformes) de l’Oligocène de Piatra neamt (Roumanie). Oryctos 4, 17–38.
Campbell, M.A., Chanet, B., Chen, J.-N., Lee, M.-Y., Chen, W.-J., Submitted. Origins and relationships of the Pleuronectoidei: Molecular and morphological analysis of living and fossil taxa.
Chanet, B., 1994. Eubuglossus eocenicus (Woodward 1910) from the Upper Lutetian of Egypt, one of the oldest solejds (Teleostei, Pleuronectiformes). Neues Jahrb. Für Geol. Paläontol. Abh. 391–398.
Gaudant, M., Gaudant, J., 1969. Note sur un pleuronectiforme nouveau conserve au Service geologique de Tunisie; Numidiopleura enigmatica nov. gen., nov. sp. Bull. Société Géologique Fr. S7-XI, 660–665.
Gibson, R.N., Nash, R.D., Geffen, A.J., Van der Veer, H.W., 2015. Flatfishes: biology and exploitation, 2nd ed. John Wiley & Sons, Chichester, UK.
Hensley, D.A., 1997. An overview of the systematics and biogeography of the flatfishes. J. Sea Res. 37, 187–194.
Hensley, D.A., Ahlstrom, E.H., 1984. Pleuronectiformes: Relationships, in: Moser, H., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W., Richardson, S.L. (Eds.), Ontogeny and Sytematics of Fishes. American Society of Ichthyologists and Herpetologists, Lawrence.
Hoshino, K., 2001. Monophyly of the Citharidae (Pleuronectoidei: Pleuronectiformes: Teleostei) with considerations of pleuronectid phylogeny. Ichthyol Res 48.
Kaschner, K., Kesner-Reyes, K., Garilao, C., Rius-Barile, J., Rees, T., Froes, R., 2015. AquaMaps: Predicted range maps for aquatic species. [WWW Document]. AquaMaps. URL www.aquamaps.org (accessed 8.8.16).
Nelson, J.S., 2006. Fishes of the World, 4th ed. John Wiley & Sons, Inc, Hoboken, New Jersey.
Pardo, B.G., Machordom, A., Foresti, F., Porto-Foresti, F., Azevedo, M.F., Bañon, R., Sánchez, L., Martínez, P., 2005. Phylogenetic analysis of flatfish (Order Pleuronectiformes) based on mitochondrial 16s rDNA sequences. Sci. Mar. 69, 531–543.
Woodward, A.S., 1910. On a fossil sole and a fossil eel from the Eocene of Egypt. Geol. Mag. 7, 402–405.